English
.
The Dutch national health care costs for coronary artery disease (CAD) amounted 2.4 billion euros in 2015 and are expected to increase to 4.2 billion euros in 2030 [1]. Currently, the usual diagnostic strategy includes a variety of invasive and noninvasive tests that are being used interchangeably at the discretion of the treating cardiologist. Despite these tests, many patients with chest pain due to CAD are misdiagnosed with non-cardiac chest pain and account for one third of patients who subsequently endure a myocardial infarction [2].
Recent single-approach trials have demonstrated the potential of a uniform diagnostic and treatment scheme that is likely to improve efficacy as well as lower costs. In the SCOT-HEART trial, the use of Computed Tomography Coronary Angiography (CTCA) as additional diagnostic test increased the frequency and certainty of CAD diagnosis due to the improved detection of both non-obstructive and obstructive CAD. The subsequent targeted treatment with preventive pharmacotherapy resulted in a significant reduction of myocardial infarctions at 5-year follow up [3, 4]. At the same time the ISCHEMIA trial has shown that optimal medical therapy (OMT) in patients with obstructive CAD and proven ischemia was non-inferior to an early invasive revascularization strategy [5].
Based on these trials, we hypothesize that a diagnostic strategy consisting of CTCA to detect CAD and initiate OMT, with angiography and revascularization only in patients with refractory angina and proven ischemia by functional imaging, will result in superior clinical outcomes and reduced health care costs. We will investigate this hypothesis in the CLEARCAD trial.
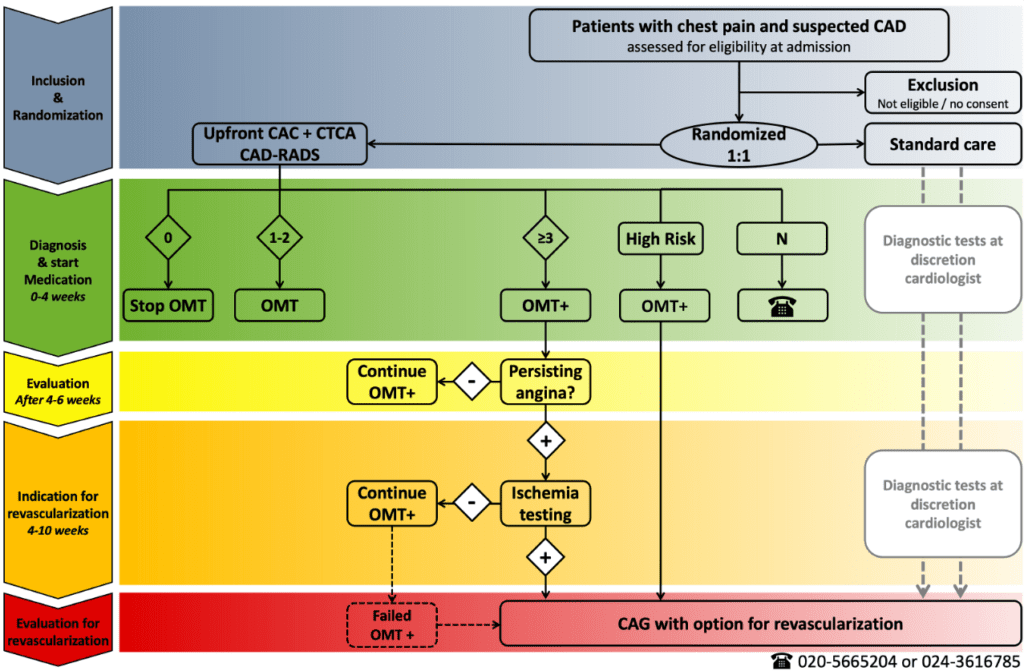
The CLEAR-CAD trial is a randomized clinical multicenter trial in the Netherlands in patients with suspected CAD, comparing usual care with a staged, uniform diagnostic and treatment strategy guided by upfront CTCA. In the intervention arm, based on CTCA, patients are categorized into three groups: (1) no CAD: these patients will not be treated and can be reassured, (2) non-obstructive CAD: these patients will be treated with preventive OMT, and (3) obstructive CAD: these patients will be treated with preventive OMT and, if chest pain persists, they will undergo additional diagnostic testing for ischemia detection. Furthermore, if in these latter patients substantial myocardial ischemia on non-invasive functional imaging is detected, then they will undergo invasive coronary angiography with a direct option for revascularization. In the control arm, usual care will be according to the treating physicians, currently characterized by a diversity of invasive and non-invasive diagnostics.
The trial is designed to demonstrate superiority regarding clinical outcomes at 3-year follow-up, and superiority regarding cost-effectiveness. Secondary outcomes will include anginal complaints. In order to demonstrate superiority regarding clinical outcomes, around 6,500 patients will be included.
CLEAR-CAD is designed by a collaboration of experts and members of the Dutch Societies of Cardiology, Radiology, Nuclear Medicine and Thoracic Surgery. This project is funded by ZonMw under the auspice of the national Health care and Appropriate care (ZE&GG) program and Zorgverzekeraars Nederland.
ZE&GG is a collaborative health care program of the Federatie Medisch Specialisten, Verpleegkundigen & Verzorgenden Nederland, Zorgverzekeraars Nederland, Nederlandse Federatie van UMCs, Nederlandse Vereniging van Ziekenhuizen, Zelfstandige Klinieken Nederland, Patiëntenfederatie Nederland and the Ministry of Health [6]. It will be one of the larger trials on the diagnostic and treatment pathway in suspected CAD. We expect the results to drive the recommended care for CAD, and to be able to start patient inclusion in 2022.
Inclusion criteria
· Chest pain, CAD suspected
· ≥18 years of age
Exclusion criteria
· ACS (STEMI, NSTEMI, unstable AP)
· History of ACS (incl unstable AP)
· CAD on previous imaging
· History of PCI or CABG
· Persistent atrial fibrillation
· Severe renal function impairment (eGFR <30ml/min)
· Severe allergic reaction to Iondine contrast
· Pregnancy
· Life expectancy < 1 year
References
1. Nederland Z. Verbetersignalement Pijn op de borst (verdenking) stabiele angina pectoris. 2017
2. Sekhri N, et al. How effective are rapid access chest pain clinics? Prognosis of incident angina and non-cardiac chest pain in 8762 consecutive patients. Heart. 2007 Apr;93(4):458-63
3. SCOT-HEART Investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015 Jun 13;385(9985):2383-91
4. SCOT-HEART Investigators. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N Engl J Med. 2018 Sep 6;379(10):924-933
5. Spertus JA, et al. Health-Status Outcomes with Invasive or Conservative Care in Coronary Disease. N Engl J Med. 2020 Apr 9;382(15):1408-1419
6. zorgevaluatiegepastgebruik.nl
Adres
Amsterdam UMC,
locatie AMC, Meibergdreef 9,
1105 AZ Amsterdam
Telefoon
020-5665204
clearcad@amsterdamumc.nl